The Cellular Secret to Resisting the Pressure of the Deep Sea

Quanta Science Podcast
Deep Dive
深海生物的压力适应:细胞膜脂质的奥秘
我长期研究细胞膜的生物化学。我们知道,地球上的生物体为了适应不同的环境,进化出了各种各样的机制。然而,对于深海生物如何在分子水平上应对极端高压环境,我们知之甚少。最近发表在《科学》杂志上的研究,让我们对这个问题有了更深入的理解。这项研究的核心发现是:深海生物细胞膜中的脂质,与浅海生物有着显著的不同,而这种差异正是它们适应深海高压环境的关键。
这项研究的突破,源于对栉水母(comb jellies)细胞膜的深入研究。栉水母是一种结构简单的海洋生物,广泛分布于各种海洋环境,从浅海到深海都有它们的踪迹。我们与深海生物学家Steve Haddock合作,收集了来自不同深度的栉水母样本,包括来自蒙特雷湾浅海和12000英尺深海的样本,以及来自北极寒冷浅海的样本。
研究发现,深海栉水母细胞膜中含有大量磷脂酰乙醇胺(plasmalogens),这是一种曲型脂质,在浅海生物中含量相对较少。在标准大气压下,磷脂酰乙醇胺分子呈现弯曲的形状,类似于羽毛球的球拍。然而,在深海高压环境下,其尾部会相互挤压,形成一种既稳固又具有流动性的细胞膜结构。我们称这种新型的适应机制为“同曲率适应(homeocurvature adaptation)”。
为了验证这一发现,我们利用基因工程技术,改造了大肠杆菌,使其细胞膜中产生更多或更少的磷脂酰乙醇胺。实验结果表明,含有更多磷脂酰乙醇胺的大肠杆菌,在高压环境下具有更强的耐受性。这有力地证明了磷脂酰乙醇胺在深海生物高压适应中的关键作用。
这项研究的意义远不止于此。深海环境是地球上最大的生物圈之一,理解深海生物的适应机制对于我们认识生命本身至关重要。此外,磷脂酰乙醇胺也存在于人类大脑等组织中,其含量变化与阿尔茨海默病等神经退行性疾病相关。因此,这项研究的发现,也为相关疾病的研究提供了新的思路。
值得注意的是,来自北极寒冷浅海的栉水母,并没有表现出高含量的磷脂酰乙醇胺。这表明,深海生物的脂质适应机制,是针对高压环境的特殊进化,而非单纯的低温适应。
目前,我们正在进一步研究这种脂质适应机制在其他深海生物,例如生活在深海热液喷口附近的生物中的普遍性。我们也对古菌(archaea)的脂质适应机制很感兴趣,因为古菌的脂质与细菌和真核生物的脂质在化学性质上有所不同,但它们是否遵循相同的物理学原理,还有待进一步研究。
总而言之,这项研究揭示了深海生物细胞膜脂质的独特适应机制,为我们理解生命在极端环境下的生存策略提供了新的视角,也为相关生物医学研究开辟了新的方向。 这项研究不仅拓展了我们对深海生物的认知,也加深了我们对细胞膜结构与功能的理解,并为未来研究提供了新的方向和启示。
- Deep-sea environment is characterized by extreme pressure, cold temperatures, and darkness.
- Little is known about how cells and molecules adapt to deep-sea pressure.
- Itai Budin and Steve Haddock collaborated to study the role of lipid molecules in deep-sea adaptation using comb jellies.
Shownotes Transcript
Welcome to the "Quanta Science Podcast." Each episode we bring you stories about developments in science and mathematics. I'm Susan Vallett.
The bottom of the ocean is cold, dark, and under extreme pressure. It is not a place suited to the physiology of us surface dwellers. At the deepest point, the pressure of 36,200 feet of seawater is greater than the weight of an elephant on every square inch of your body. Yet Earth's deepest places are home to life uniquely suited to these challenging conditions.
So how do organisms survive there? That's next. Quantum Magazine is an editorially independent online publication supported by the Simons Foundation to enhance public understanding of science.
Scientists have studied how the bodies of some large animals, such as anglerfish and blobfish, have adapted to withstand the pressure of the deep sea. But far less is known about how cells and molecules stand up to the squeezing, crushing weight of thousands of feet of seawater.
Itai Budin studies the biochemistry of cell membranes at the University of California, San Diego. We know that organisms specialize for different environments on Earth. We know that the animals that live down in the deep sea are not ones that live in surface waters. They're not ones you'll see in an aquarium. They're clearly biologically specialized, but we know very little at the molecular level
what is actually determining that specialization, if anything at all. In a recent study published in Science, researchers took the deepest look yet at how cells have adapted to life in the abyss. In 2018, Budin met Steve Haddock, a deep sea biologist. They combined forces to investigate whether the lipid molecules that cell membranes are made of could help explain how animals have come to thrive in such a high pressure environment.
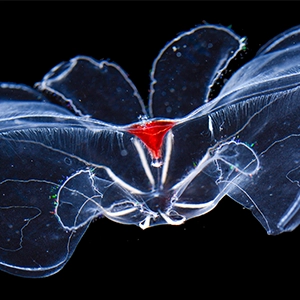
To find out, they turned to comb jellies, the simple, delicate animals that Haddock studies at California's Monterey Bay Aquarium Research Institute.
Douglas Bartlett studies how microbes sustain life at depth and pressure at the University of California, San Diego. He wasn't involved in the new study. These comb jellies are these beautiful pelagic marine animals, iridescent, and oftentimes if you've seen videos of them in the ocean, you see the combs for which they're named, beating, and that's how they were able to swim in the ocean. So they're quite striking animals, very cool looking animals.
Haddock's student, Jacob Winokoff, led the interdisciplinary team. They discovered that the membranes of comb jellies that reside in the depths are made of lipid molecules with completely different shapes than those of their shallow water counterparts. Three-quarters of the lipids in the deep-sea comb jellies were plasmalogens, a type of curved lipid that is rarer in surface animals.
In the pressure of the deep sea, the curvy molecule conforms to the exact shape needed to support a sturdy yet dynamic cell membrane. Here's Bartlett again. We're talking about the largest part of the biosphere, the deep ocean, the cold deep ocean. It was important to try and understand what makes the organisms in the deep sea different from those in, say, polar environments. So this paper comes along and they provide a whole other explanation of
for how the lipids of deep sea animals and likely deep sea microbes are adapted in a way that's pressure specific. It's an amazing paper. It goes into a really detailed lipid structural study with quite profound implications.
Saul Gruner researches molecular biophysics at Cornell University. He was consulted for the study, but isn't a co-author. We look at our world and there's a tendency to think that, well, we knew where life on the planet was and we understood how it behaved under a variety of conditions and where it came from. And in fact, that's not true. It's never really been true. There's so much to learn. So the broadest significance of
Jacob and I study is that they are looking into an area that I think to a large degree has not been explored. Plasmodium lipids are also found in the human brain, and their role in deep sea membranes could help explain aspects of cell signaling. More immediately, the research unveils a new way that life has adapted to the most extreme conditions of the deep ocean.
The cells of all life on Earth are encircled by fatty molecules known as lipids. Here's Winokoff. Membranes are made up of these lipids that have the watery head group and these two greasy tails made of carbon and hydrogen, and they line up back to back. And if you put some lipids in a test tube and you add water, they will line themselves up that way all on their own. And it's just like oil and water, oil and vinegar separating in a dish because like wants to be with like. So the greasy ends separate.
come together and the water loving end of the molecule faces outwards. That basic plan where you have one side of the molecule being greasy and the other side being water loving is universal to lipids and it's what makes them work.
For a cell, an outer lipid membrane serves as a physical barrier that, like the external wall of a house, provides structure and keeps a cell's insides in. But the barrier can't be too solid. It's studded with proteins, which need some wiggle room to carry out their various cellular jobs, such as ferrying molecules across the membrane. And sometimes a cell membrane pinches off to release chemicals into the environment and then fuses back together again.
So for a membrane to be healthy and functional, it must be sturdy, fluid, and dynamic at the same time. Here's Winokoff again. The membranes are balancing right on the edge of stability, and that is exactly where they want to be. The kind of crazy thing about a lipid membrane...
that I found mind-bending when I learned it, is that even though it has this really well-defined structure, these two layers, all the individual molecules that make up the sheets on either side, they're flowing around each other all the time. It's actually a liquid crystal. So it has a higher order structure, but the molecules are moving.
Winokoff says one of the emergent properties of this structure is that the middle of the membrane is highly sensitive to both temperature and pressure, much more so than other biological molecules such as proteins, DNA, or RNA. If you take a membrane and you keep the composition the same, so you're not changing any of the lipids that are in it, and you cool it down, the molecules will move slower and slower, and then eventually they'll just lock together. It's like when you put olive oil or butter in your fridge.
Biologically, that's generally a bad thing. Like at that point, metabolic processes can't continue because the molecules aren't moving. The membrane can crack, leak. To avoid this, many cold-adapted animals have membranes composed of a blend of lipid molecules with slightly different structures to keep the liquid crystal flowing, even at low temperatures.
Winokoff says previous studies had shown that. Colder things use more unsaturated fatty acids, and that helps the lipids flow at lower temperatures. Because high pressure also slows a membrane's flow, many biologists assumed that deep sea membranes were built the same way.
But it turns out these researchers weren't getting the full picture. It would take an unexpected collaboration between biochemists and marine biologists and more advanced technology to see that deep-sea membranes had evolved a different way of going with the flow.
Comb jellies, or ctenophores, are voracious predators in fragile bodies. They are the largest animals that swim with cilia, which are lined up in rows known as combs, and they feed on a wide range of prey. Genetic evidence suggests that they were the first organisms to branch off the animal tree on their own evolutionary path.
Though they resemble jellyfish in some ways, humans are actually more closely related to jellyfish than tino-phores are. And they have successfully colonized all kinds of ocean habitats, from surface waters to ocean trenches, and from the tropics to the poles. You would expect such a wide-ranging group to be adaptable, and indeed comb jellies from the deep are built differently than those that live near the ocean's surface.
Here's marine biology researcher Douglas Bartlett, whom we heard from earlier. For the purposes of this study, they collected shallow and deep guise. You collect the deep guise and you bring them up to the surface and they just fall apart. They just melt away. Really quite dramatic. And then in the case of the shallow guise, you pressurize them. So you expose them to something more like a deep sea surface.
condition. And those combs that beat to help them to move, they beat faster and faster and faster as they get pressurized. And that movement of their cilia causes them to eventually die. But no one really knew the molecular differences that separated them.
In 2018, Haddock, an expert on comb jellies, attended a conference on the origin of eukaryotes. After watching Budin present research on cell membranes' response to temperature, he approached the lipid expert. Haddock had a graduate student, Winokov, who wanted to study adaptations to extreme pressure. It was known that lipids are sensitive to pressure, so cell membranes were a prime target for investigation. They decided to collaborate.
Haddock, Budin, and Winokoff started by collecting comb jellies from different parts of the ocean. Here's Winokoff. The shallow ones were mostly collected by scuba diving. I was on most of those dives, got to catch most of my own specimens, which is really rewarding to be able to take something from, you know, a jar in your hand through a measurement coming out of a particle accelerator. Winokoff carefully captured comb jellies from Monterey Bay's surface waters.
From one of the Monterey Bay Aquarium Research Institute's oceanographic vessels, he helped operate a deep-sea robot to collect comb jellies from depths of 12,000 feet. To control for the effects of the cold temperatures in the deep sea, he and Budin asked friends who were on their own expedition to gather surface comb jellies from frigid Arctic waters. In total, the team collected 66 animals from 17 related species.
By the time the molecular part of the project was set to begin, the pandemic had hit. So Winokov set up an experiment in his garage. Because we weren't allowed into the institute. Using a fluorescent spectrometer, Winokov sent rays of ultraviolet light into test tubes filled with small globs of membrane material from the creatures they'd collected. The fluorescent output signal, over the course of the couple of hours it took to do the experiment,
With some animals it was fine, but with the really deep sea animals, which were what we really, really wanted data from because nobody had data from them before, the signal would just go like... The results puzzled him. The deep sea membranes didn't become more fluid as he raised the temperature. That's a response considered universal among lipid membranes. And so we talked to this physicist from Cornell about this named Saul Gruner. And he said, well, you know,
Sometimes lipids are lying flat in these membranes, but under certain conditions they can form other shapes. Gruner, the former director of Cornell's particle accelerator, told them if they really wanted to know what was happening in the membranes, they'd need powerful high-energy X-rays. And he knew the perfect source.
Buried 50 feet beneath the main athletic fields at Cornell is a synchrotron, a particle accelerator that uses a high-frequency electric field and a low-frequency magnetic field to speed up charged particles. Part of the facility, which Gruner fought to establish, may as well have been designed for studying deep-sea cell membranes. A small angle X-ray scattering operation opened in 2020.
it can not only distinguish the finer details and shapes of molecules such as lipids, but also increase and decrease the pressure they're under. The team experienced some pressure too, as they had to endure late nights to make the most of their limited time at the facility. The powerful x-rays they shot at their lipid samples revealed the clearest picture yet of cell membranes from the abyss.
The deep-sea comb jellies had membrane lipids that, at our standard atmospheric pressure, have a curvier shape than those in surface cell membranes.
The animals had especially increased production of a group of lipids known as plasmalogens, says Winokoff. In these deep-sea calm jellies, they can make up three-quarters of all the lipids. And we're talking about all the lipids in the entire body of the animal, which is kind of crazy. We did a lot of checks to make sure that wasn't a mistake. Winokoff says at the surface, a plasmalogen has a small phosphate head and a pair of wide, flaring tails resembling a badminton shuttlecock.
But at high pressure, the tails squeeze together to form the necessary sturdy yet dynamic structure. Here's Budin. They start their lipids at a different spot or a different shape. So when you compress them, they still maintain kind of the right Goldilocks shape that you see in our own cells, but at these extreme pressures. Budin and Winokov named this novel modification homeocurvature adaptation.
Bartlett says taking a plasmalogen membrane to the deep sea is like pushing down on a spring. Then when things come up and the pressure is released, all of that tension on the springs are released and the spring just, you know, extends dramatically. That's when you can imagine the cells, their membranes falling apart. Meanwhile, if a surface membrane with straighter lipids is brought down to the deep, it compresses too much and becomes too rigid to function properly.
Notably, curvy plasmalogens were not present in comb jellies from the cold, shallow waters of the Arctic. Peter Meikle is a lipid biologist who works on plasmalogens at the Baker Heart and Diabetes Institute in Australia. He wasn't involved in the study. The composition of the membrane almost restricts the organisms to a particular pressure range.
So then if they come up to too low a pressure, then because they've got a particular composition of the membrane, the membranes won't function, or if they go too deep, they won't function. Budin wanted to see these lipids in action, and something occurred to him during a late session at the synchrotron. In the middle of the night when you're deliriously tired, I mean, it is, yeah, sometimes it does take. Budin stumbled across a paper with an intriguing approach to studying lipids,
The authors had engineered E. coli bacteria to produce plasmalogens in their membranes instead of their normal lipids.
Budin realized that his team could similarly coax the bacteria to produce more plasmalogens and pressurize them to see how the membranes held up in living cells. To test the hypotheses we had about pressure tolerance and pressure specialization, we actually used E. coli cells and we engineered the lipids of E. coli cells and we made the E. coli lipids either more of a highly curved shuttlecock or less.
And we saw how the E. coli cells would respond under pressure in the lab because we could put E. coli cells and pressure chambers in the lab and look at this. Following the paper's methods, they showed that the bacteria with plasmologen membranes could indeed better tolerate pressure than typical ones. Winokoff says these experimental membranes were made up of only 20% plasmologens, but it was enough to make a difference.
Bartlett was impressed that the effect of the curved lipid shapes occurred in such unrelated species. What is likely to come out of this is that we'll find that this principle of homeocurvature adaptation will become a universal property of life. And that in addition to thinking about how membranes function,
adjust their viscosity, they're going to have to adjust their curvature as well for the various things that membranes do. And so that'll result in a different way of thinking about membranes. And I don't know where all that's going to lead. Plasmalogens aren't limited to the deep sea. They're also found to varying degrees in other organisms, including humans. The percentage of plasmalogens within humans depends on the cell type.
In the liver, plasmalogens make up 5% of phospholipids. In muscles, they can range between 20% and 40%. And in the brain, they make up about 60%. In fact, the deterioration of plasmalogens has been linked to neurodegenerative disorders such as Alzheimer's disease.
You remember Peter Meikle from earlier. He studies plasmalogens because of their links to mammalian health. From my point of view, at least, the evidence suggests that the plasmalogens are more protective. Winokoff speculates that plasmalogens might give nerve cells the right flexibility for their communication needs.
To send signals, neurons fill cellular sacs with neurotransmitters. Then those sacs fuse with the cell membranes to release the signaling compounds onto the next neuron. Winnikoff suggests that maybe plasmodium's curvy structure makes that possible.
Mikko likes the idea. Certainly they're the primary sort of cone shape that allow membranes to form those types of curvatures. As studies better understand the role of lipids in membrane function, the findings could be relevant for a broader range of membranes. Here's Saul Gruner. They've opened up more questions than they've answered, but hopefully it will catalyze people to start thinking about
doing more experiments, going deeper into the subject. Indeed, Winokoff, who's now a postdoctoral fellow at Harvard University, is looking into how universal this lipid adaptation mechanism is across different organisms.
He's started experiments to figure out whether organisms found at hydrothermal vents, deep ocean areas where magma and seawater meet, have similar adaptations. He says it would be really interesting to look at archaea, the third branch of life. Archaea lipids behave differently than those found in bacteria and eukaryotes. Winokoff says they follow different chemistry, but he wonders, do they follow the same physics?
Arlene Santana helped with this episode. I'm Susan Vallett. For more on this story, read Yasmin Saplakoulou's full article, "The Cellular Secret to Resisting the Pressure of the Deep Sea," on our website, quantamagazine.org. Just a note, Itay Budin has received funding from the Simons Foundation, which also funds the editorially independent Quanta Magazine and Quanta Science podcast. Simons Foundation funding decisions have no influence on our coverage.
Explore math mysteries in the Quanta book, The Prime Number Conspiracy, published by the MIT Press. Available now at Amazon.com, BarnesandNoble.com, or your local bookstore.